The Volume of an Ideal Gas Is Zero at
An ideal gas initially at p 209 atm V 0750 L and T 297 C is expanded under constant pressure to a final volume of 292 L. For most gases there is a linear relationship between temperature and pressure see gas laws ie gases contract indefinitely as the temperature is decreased.
- ΙπΙ ΑΣΦ.

. Although this seems a paradox there is one fact that does not necessarily cause problems with this method of calculation. But no gas is ideal and real gases show all sorts of non-ideal behaviour. The molar volume of a gas at STP is _____ L.
One bar 100 000 Pa. Using the state equation for an ideal gas. In actuality all gases condense to solids or liquids well above this point.
Gases are made out of molecules and they can never have a zero volume. At the same time the pressure of the gas triples. Correct answer to the question What is the volume of 0200 mol of an ideal gas at 200 kpa and 400 k.
The fact that ideal gas particles dont change their position at zero temperature is irrelevant because thats not how entropy is defined. The molar volume at STP is 22414 liters so using the ideal-gas law PV nRT the volume at 1 bar at 0 C is 101 325 100 000 22414 L 22711 L. Although the actual volume of an ideal gas will be zero at 0 K why is the volume of air not zero at this temperature.
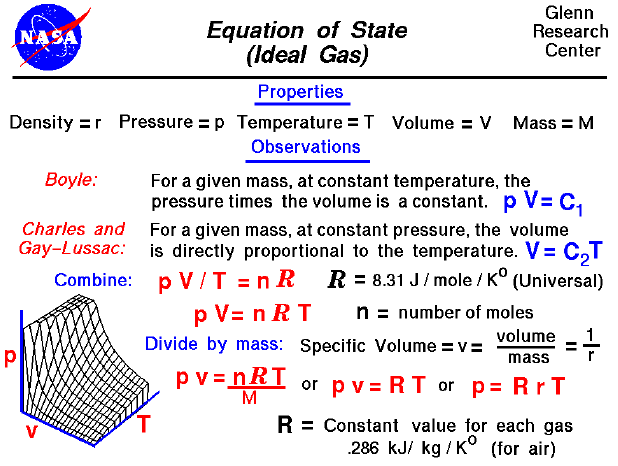
T the thermodynamic temperature will be equal to 0 so the product of the molar gas constant R 831 JmolK and the amount of moles n will also be zero. Theoretically at absolute zero the volume of an ideal gas would be zero and all molecular motion would cease. Where p is pressure V is volume and n is number of moles R is universal gas constant and T is temperature in kelvin.
Absolute temperature is temperature measured using the Kelvin scale where zero is absolute zero The zero point is the temperature at which particles of matter have their. If the gas had not turned into a liquid the -27315 C will be the temperature the gas volume will become zero. Standard temperature and pressure STP in the context of gases refers to _____.
At a fixed temperature and pressure the volume occupied by a gas is _____ proportional to the number of moles of gas present. Chemistry questions and answers. However if we extend the original line dotted part of line to the temperature axis you will notice that it crosses the temperature axis at -27315 C.
The volume of an ideal gas is zero at _____. Theoretically the volume of an ideal gas molecule at absolute zero gas would be zero thus all the molecular motion would cease. The volume of a definite amount of hydrogen gas taken at 2 a t m pressure and 2 5 o C temperature is 4 0 0 m l.
A Suggest a method to increase the volume of this gas without change in pressure. For example real gases liquify then solidify as the temperatue falls. A 06343T b 06334T c 43T d 34T.
We know that 0 K is 27315 C which is nothing but the Absolute zero temperature. Also the gas will no longer be a gas at absolute zero but rather a solid. A theoretical coldest temperature that can be approached but never reached.
Part A - What is the final temperature of the gas. Absolute zero is zero on the Kelvin scale -27315C on the Celsius scale and -45967F on the Fahrenheit scale. At zero temperature this is equal to the number of lowest-energy states of the system.
Such a gas can be used to define the thermodynamic temperature scale with its zero where P and V drop to zero. As the gas is cooled it. If the original absolute temperature of the gas was T that is the new temperature after compression.
B If the volume of the gas is changed to 2 0 0 m L what will be the new pressure. The temperature at which the volume of an ideal gas becomes zero. Temperature is constant c State the gas law used to.
But in actual all gases condense to solids or liquids well above this point. The implication is that at absolute zero temperature the volume of a gas would similarly be zero at which point it is non-existent. Of the following only _____ is impossible for an ideal gas.
This is the lowest-energy state for an ideal gas. Gas molecules repel each other more at low temperatures. 27315K and 1atm.
T Submit Request Answer Part B - How much work is done on the gas. W J Submit Request Answer. Therefore the product of PV must be zero also.
At such low temperatures gases assume non-traditional states the Bose-Einstein and fermionic condensates. As you can see it turns into a liquid at a volume value higher than zero. The ideal gas law is approximately true for most gases and as its name implies is exactly true for an ideal gas an imaginary gas which obeys Boyles law perfectly.
From the above graph we can see that at T 27315 C Volume become zero. For ideal gases under these conditions equal _____ of gas contain equal numbers of particles or moles. According to gas equation pVnRT.
So here p 1 bar n 1 mol T 273 K R 008314 L barK mol. At constant pressure the volume of an ideal gas is given by Charles law. However in real life gas is matter.
Gases become liquid at very low temperatures. The volume holding 063 mole of an ideal gas is compressed to 14 of its original volume by a piston. So putting values in the formula 1.
Absolute zero as a temperature itself is non-existent. 30 The volume of an ideal gas is zero at 30 A -273 C в 0 C C-273 K D-45 F E -363 K. When T 0 K Volume is zero.
Gases expand as the temperature decreases. Entropy is the number of possible configurations of a system. The pressure of the gas must be zero or volume of the gas must be zero.
And this law tells us that when the temperature T falls to zero the volume V also becomes zero. In practice we know that there is a small amount of energy at absolute zero the so-called zero-point. If an ideal gas is cooled to absolute zero its volume would also be zero.
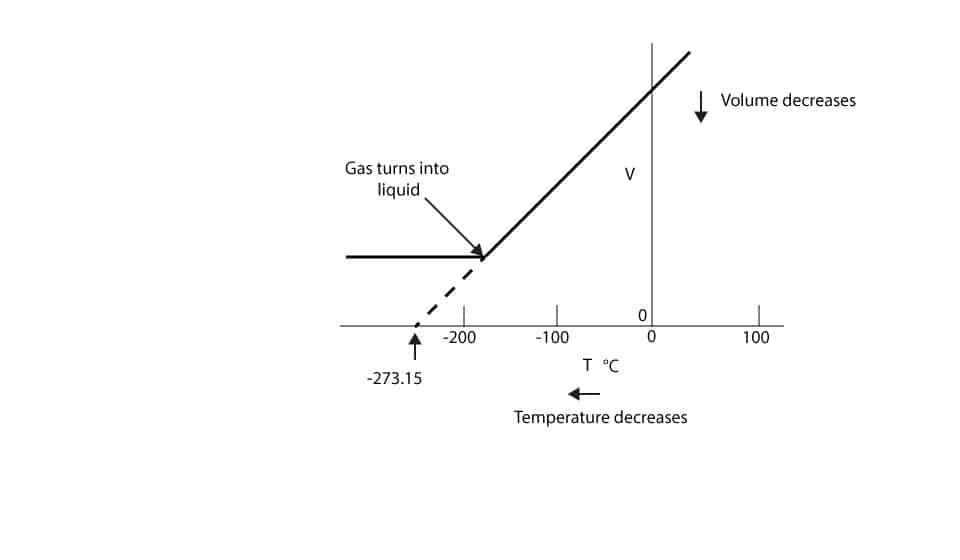
Why Can T The Volume Of Gas Become Zero As Its Temperature Decreases

Ideal Gas Law Brilliant Math Science Wiki

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry I
0 Response to "The Volume of an Ideal Gas Is Zero at"
Post a Comment